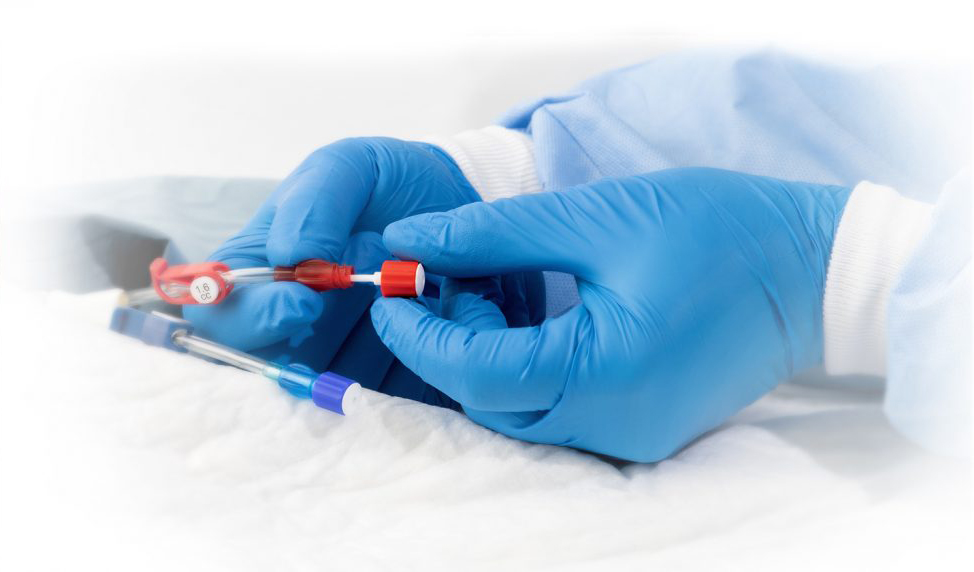
ClearGuard™ HD
Antimicrobial Barrier Caps
The proven solution for reducing bloodstream
infections in haemodialysis catheters
The ClearGuard™ HD cap is the first and only device designed to kill infection-causing bacteria inside a haemodialysis catheter hub.
Protects haemodialysis catheters
The ClearGuard™ HD caps are easy to use, yet highly effective and clinically proven to reduce central line-associated bloodstream infections (CLABSIs) in haemodialysis catheter patients.
*Designed to kill microorganisms, not intended to be used for treatment of existing infections.
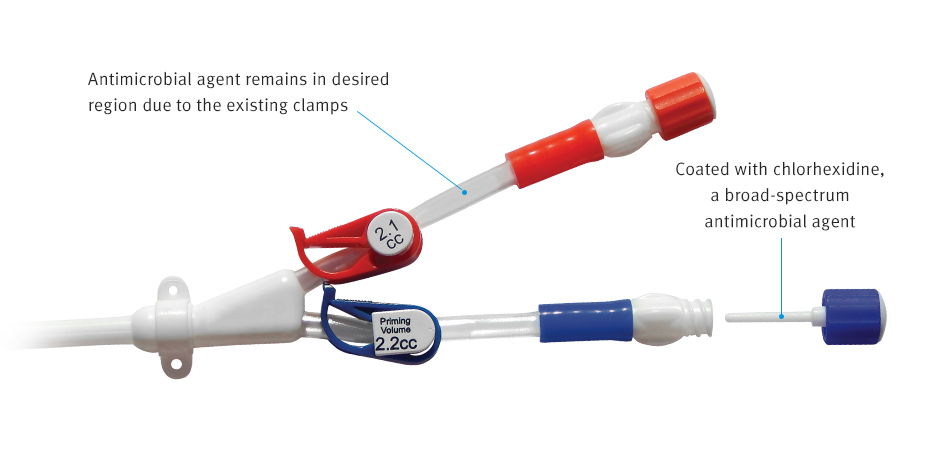
A simple, intuitive design
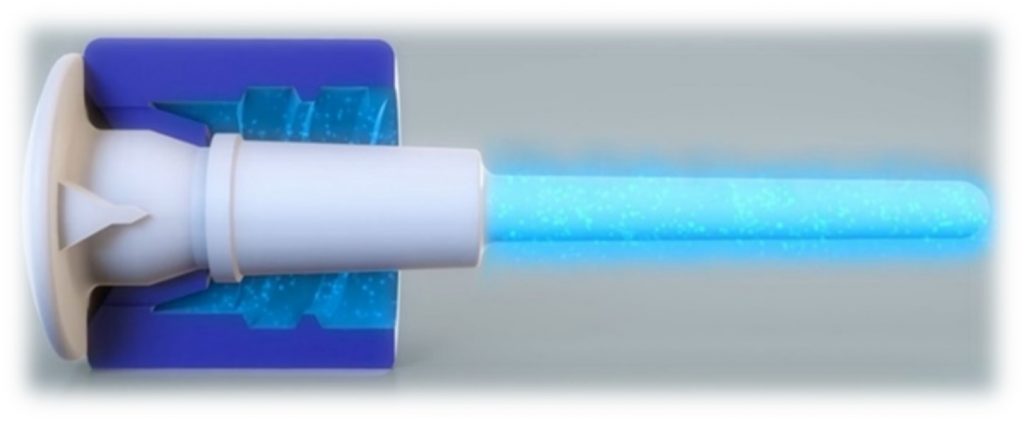
The ClearGuard™ HD cap features a rod that extends into the haemodialysis catheter hub. The rod and cap threads are coated with chlorhexidine, a well-known broad-spectrum antimicrobial agent.
ClearGuard™ HD caps are a proven solution
- When the ClearGuard™ HD cap is inserted into a liquid-filled catheter, chlorhexidine elutes from the rod into the catheter lock solution
- The chlorhexidine coating dissolves to kill microorganisms on the inside and outside of the catheter hub
- The existing catheter clamp holds the antimicrobial agent inside the catheter hub between treatments
- ClearGuard™ HD caps are used in place of a standard cap or connector
Clinically proven
ClearGuard™ HD caps have been clinically proven to reduce CLABSIs in haemodialysis catheter patients.
Multiple large, prospective, cluster-randomized multicentre open-label trials demonstrated a significant reduction in the rate of positive blood cultures (PBCs) and CLABSIs using ClearGuard HD caps vs. control groups.

J Am Soc Nephrol 2018 Apr; 29(4):1336-1343.
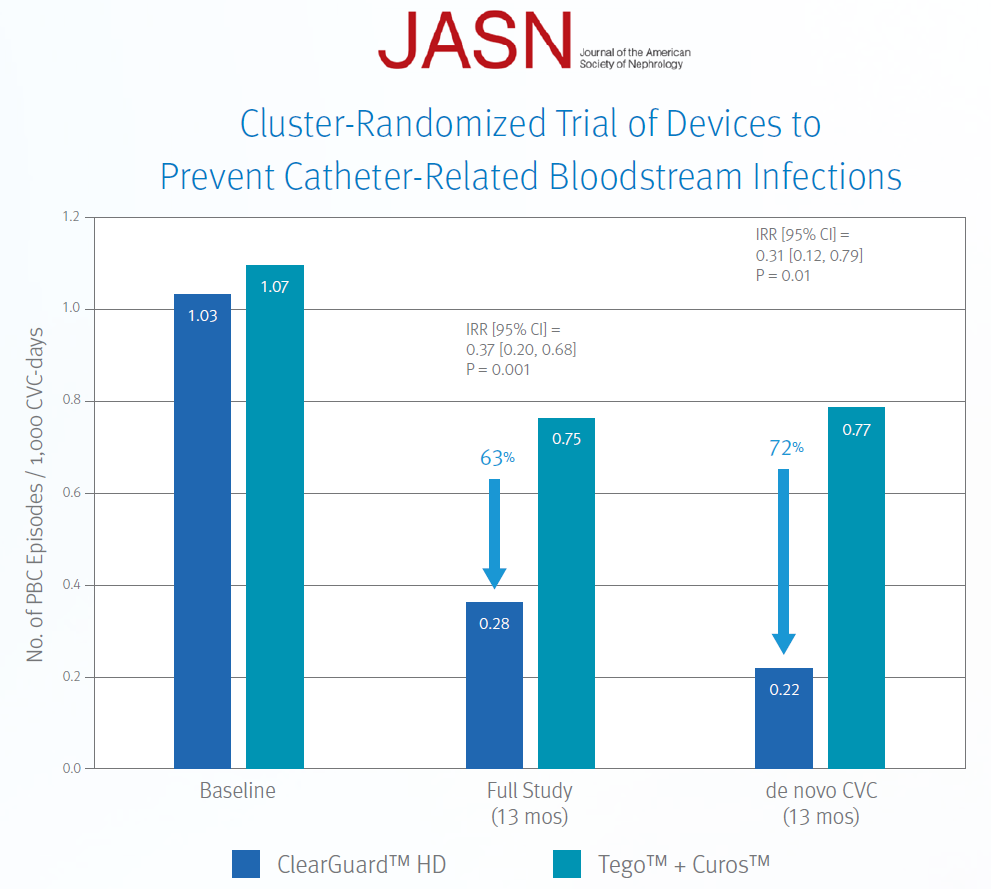
ClearGuard™ HD caps vs. Tego™+ Curos™
Brunelli, SM et al. Cluster-randomized trial of devices to prevent catheter-related bloodstream infection.
J Am Soc Nephrol 2018 Apr; 29(4):1336-1343.
- 13-month prospective, cluster-randomized, multicentre open-label trial
- 1671 patients (826 treatment, 845 control) accruing ~183,000 CVC days
- 40 centers across the US
- Primary endpoint was PBC rate as an indicator of BSI rate
Results: Use of the ClearGuard HD caps for 13 months was associated with a 63% lower BSI rate vs. use of Tego + Curos.
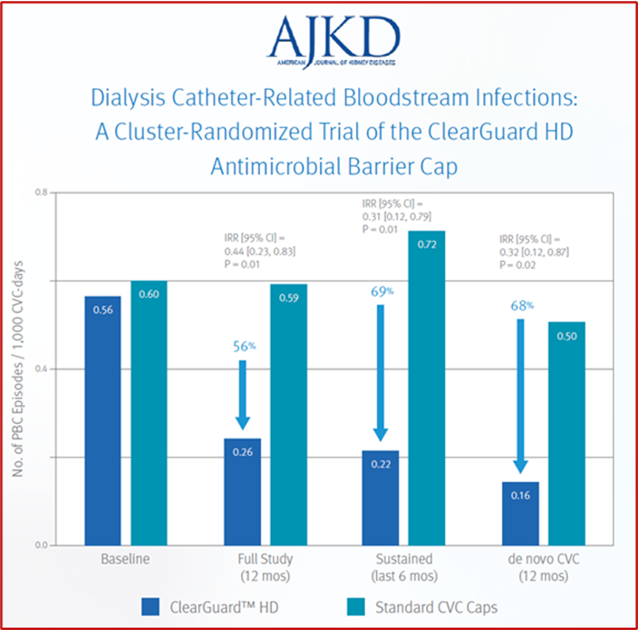
ClearGuard™ HD caps vs. standard dialysis caps
Hymes, JL et al. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard™ HD antimicrobial barrier cap. Am J Kidney Dis. 2017; 69(2):220-227.
- 12-month prospective, cluster-randomized, multicentre, open-label comparative effectiveness trial in haemodialysis patients with central venous catheters
- 2470 patients (1245 treatment, 1225 control) accruing ~350,000 CVC days
- 40 centers across the US
- Primary endpoint was PBC rate as an indicator of BSI rate
Results: Use of the ClearGuard HD caps for 12 months was associated with a 56% lower BSI rate vs. use of standard caps. When considering sustained use (defined as 6 months of the study), the intervention vs. control was associated with a 69% lower BSI rate.
A new standard of care
ClearGuard™ HD caps are an important advancement in haemodialysis infection control best practice
Find out more about ClearGuard™ HD
To discuss your needs or find out more about ClearGuard™ HD caps and how they can benefit your infection control practices for patients with CVCs, contact us at Valiant Medical today